AnaLig®: Highly Selective Solid Phase Extraction for Specific Target Analytes
AnaLig® MRT™ products and processes are designed to precisely recognize structural and electronic features of, and selectively complex with, a target analyte.
Applications of AnaLig® MRT™ products and processes include routine laboratory analyses and analysis of industrial streams on an automated real time continuous basis. AnaLig® MRT™ processes are useful for analysis of environmental samples because of their ability to rapidly and selectively separate and concentrate target metals or anions present at very low concentration levels (mg/L to µg/L), shorten analysis time, generate minimal waste and minimize the environmental consequences and carbon footprint of the analysis procedure since organic solvents and other chemicals are not used and all procedures are performed at ambient temperatures.
AnaLig® is used to eliminate matrix interferents prior to doing instrumental analysis, thus increasing detection capability. The feed solution to be analyzed is passed through the AnaLig® column and the target ion is removed selectively from the solution. Elution of the column results in a concentrated eluate solution containing the separated, pure analyte at high concentration. Analysis of the concentrated solution for the desired metal is then done using standard analytical instrumentation. The entire separation, concentration, and analysis procedure can be fully automated for continuous operation. An important feature of AnaLig® MRT™ processes is the rapidity with which the analysis can be made.
AnaLig® eliminates matrix interferents prior to doing instrumental analysis, thus increasing detection capability.
AnaLig® products are available for a wide range of metals, non-metals, and anions. These include precious metals (palladium, platinum, rhodium, iridium, ruthenium, gold, silver); radionuclides (cesium, strontium, radium, plutonium, technetium, uranium); transition metals (chromium, manganese, iron, cobalt, nickel, molybdenum, rhenium); alkali and alkaline earth metals (lithium, sodium, potassium, rubidium, cesium, magnesium, calcium, strontium, barium); toxic metals (arsenic, cadmium, mercury, lead, thallium); other metals (boron, zinc, germanium, indium, antimony, bismuth) and anions (fluoride, chloride, bromide, sulfate).
AnaLig® Advantages
- Extremely high selectivities for target analytes, even in large excesses of competing species
- Rapid flow rates, due to extremely rapid kinetics, much faster than ion exchange
- Tolerance of difficult matrices, including a wide range of solute types and concentrations, wide pH ranges
- Efficient concentration of the sample species, even from very dilute analyte concentrations
- Achievement of very large concentration factors due to high selectivities, rapid flow rates, and small elution volumes
- Elimination of analytical interferences and simplification of complex matrices.
- No addition of ions to the sample solutions, since no ions are exchanged as in ion exchange
- Minimal waste production, due to non-use of organic solvents and minimal use of reagents in a simplified system.
For more information, see our papers Application of Molecular Recognition Technology to Green Chemistry Analytical Determinations of Metals in Metallurgical, Environmental, Waste, and Radiochemical Samples and Green Chemistry Molecular Recognition Processes Applied to Metal Separations in Ore Beneficiation, Element Recycling, Metal Remediation, and Elemental Analysis.
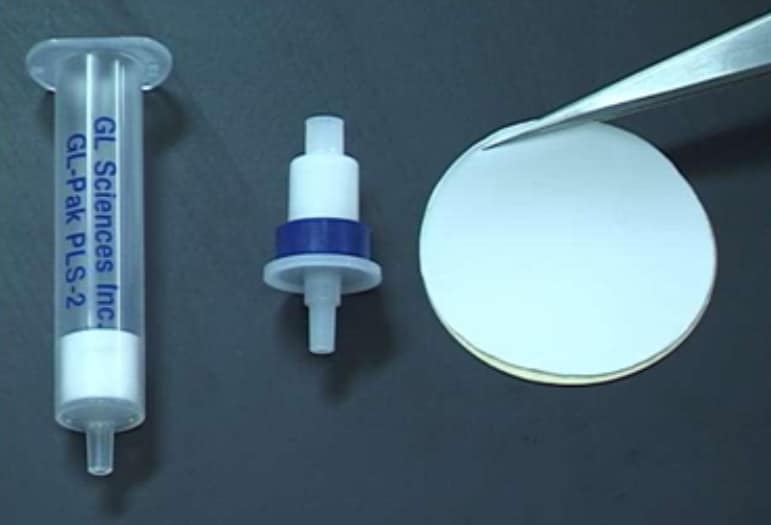
IBCs partner, GL Sciences (Japan) packs AnaLig® particles into columns or disks. The feed solution to be analyzed is passed through the column and the target ion is removed selectively from the solution. Elution of the column using a concentrated eluent solution results in concentration by a large factor of the separated, pure analyte, in the eluate. Analysis of the concentrated solution for the desired metal is then done using standard analytical instruments. The entire separation, concentration, and analysis procedure can be fully automated for continuous operation. An important feature of the process is the rapidity with which the analyses can be made, hours instead of days by conventional separation methods.
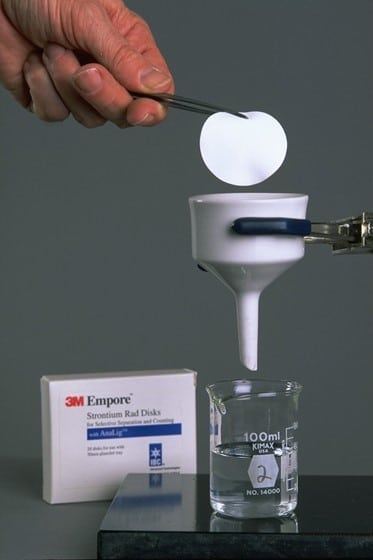
An example of the usefulness of AnaLig® is in the nuclear industry, where IBC’s separations technology is used worldwide to analyze radionuclides. The environmental and health consequences of radionuclides can be grave. They can enter the food chain and contaminate water, milk or other nutrients. Rad Disks, developed jointly by IBC, 3M and Argonne National Laboratory, have dramatically reduced the time required to analyze radionuclides such as strontium and radium (i.e., days to less than 20 minutes). In an Innovative Technology Summary Report prepared for the U.S, Department of Energy, Rad Disks were shown to produce savings of hundreds of thousands of dollars over EPA Method 905.0.
The yearly cost of radiochemical analyses involved in decontaminating Department of Energy sites now exceeds $300 million. To reduce these costs, researchers at DOE’s Argonne (Ill.) National Laboratory, 3M, St. Paul, Minn., and IBC Advanced Technologies, American Fork, Utah, have jointly developed a simpler, more cost-effective way to analyze these radionuclides.
The result is Empore Rad Disks for the selective adsorption of radioisotopes in aqueous samples. Measuring 47 mm in diameter and only 500 µm in thickness, Empore Rad Disks fit into existing instrumentation. They are made of polytetrafluoroethylene fibrils (about 10%) embedded with densely packed AnaLig® particles (about 90%). Sorption of target radioisotopes is very efficient: They are concentrated close to the surface of the disk, while non-target radioisotopes simply pass through and remain in the filtrate.”
Reprinted from R&D Magazine September, 1996 © 1996 by Cahners Publishing Company
Winner of the R&D 100 Award and the Federal Laboratory Consortium Award for Excellence in Technology Transfer, as well as being recognized as a Top 40 R&D 100 Award Winner, Rad Disks are used worldwide. Empore Rad Disks are sold by CDS Analytical.
Expert Reviews
Coha, B. et al. 2021. Synergy of flow injection system and molecular recognition technology products for rapid determination of Sr-89, 90, and Pb-210, Talanta, 225, 121959.
“These highly selective materials for Sr [AnaLig® Sr-01 and SuperLig® 620] have enabled the development and implementation of automated and miniaturised procedures, which allow more rapid procedures than conventional ones, decrease labour costs, reduce waste, and most importantly, decreases analyst exposure to radiological dose.”
“In the case of environmental monitoring, samples usually contain both Pb and Sr, therefore this procedure is suitable for their simultaneous determination. The possibility of binding Pb and Sr and simultaneously separating them using one column represents a significant improvement over existing methods; other authors use an additional column with, e.g. a cation exchanger for Pb removal”
“If the time necessary to obtain pure Sr fractions is compared, roughly two and a half days are required for the method using a combination of anion exchange resins and nitric acid in an alcohol solution around the proposed method reduces this time even further to 1–5 h, as well as additionally separating and determining Pb and Ba. Chemical recoveries were also checked by spiking the samples with Sr-85; the results showed that recovery was (99 ± 3) % in all cases.”
“Such solid phase extraction systems, based on molecular recognition technology (MRT) are highly discriminating; they have the ability to distinguish and separate target ions even if they are present in trace amounts in solutions containing higher concentrations of ions with similar chemical properties.”
“The proposed method solves major problems with obtaining a pure Sr fraction after separation using AnaLig® Sr-01 and SuperLig® 620 resins when Pb and Ba(Ra) are present in the samples.”
“The automated procedure may be easily implemented in laboratories worldwide and can also be used at sampling sites.”
“Since the automated system is small and portable, in combination with, e.g., a portable LSC [liquid scintillation counter], it can be used outside the laboratory at sampling sites to obtain even more rapid results if necessary.”
Rahman, et al. 2021. Selective separation of tri- and pentavalent arsenic in aqueous matrix with a macrocycle-immobilized solid-phase extraction system, Water, Air, & Soil Pollution, 224, 1–11.
“The process offers a single-step separation option of trivalent and pentavalent arsenic species that commonly exist in the natural aqueous matrix. Easy operation, rapid separation performance, and reusability for more than 100 cycles without loss of the analytical performance of the MRT-SPE [solid phase extraction] are some additional characteristics of the proposed process which make it a suitable and economic option for, particularly, the arsenic-prone nations suffering from natural arsenic contamination.”