ChiraLig™ Products Separate Individual Enantiomeric Species at a High Purity Level
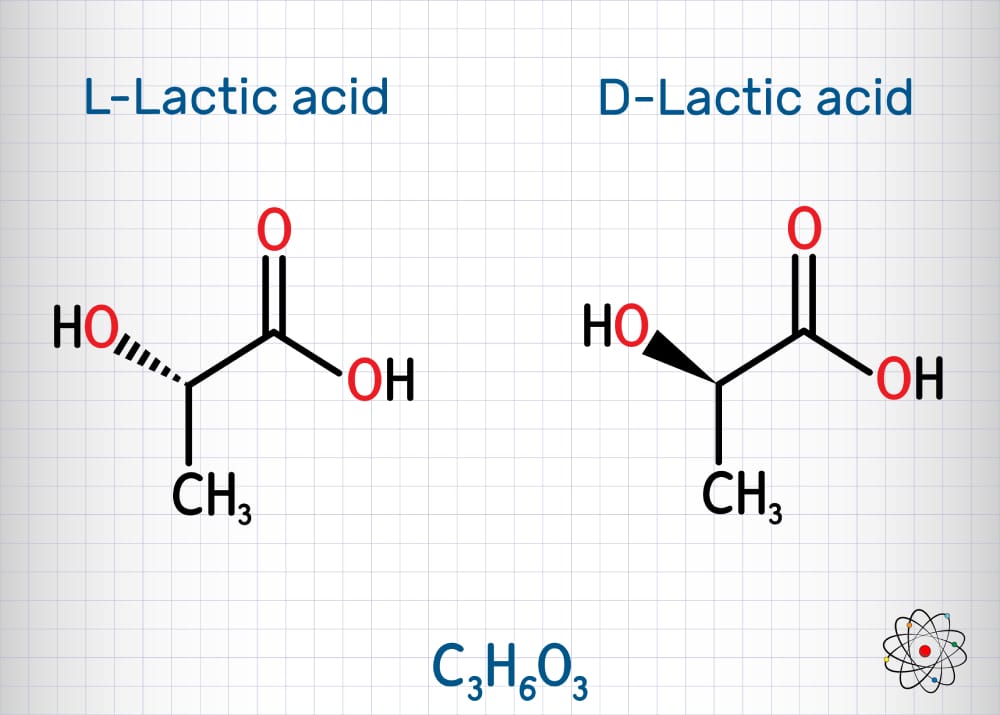
Chiral compounds consist of optically active, non-superimposable stereoisomers that are mirror images of each other (enantiomers.) This geometric property is termed chirality (from the ancient Greek for “hand”). Just as right and left hands are not superimposable, stereo-isomeric chiral molecules have the same chemical formulas but one stereoisomer is not superimposable with its mirror image. The mirror image forms of a chiral molecule can have radically different properties. The critical importance of being able to separate chiral molecules into their “right hand” and “left hand” enantiomers is illustrated by the thalidomide disaster of the late 1950s and early 1960s. The use of thalidomide, which consists of a racemic mixture of (R) (curative) and (S) (teratogenic i.e., an agent or factor which causes malformation of an embryo) enantiomers, resulted in a medical tragedy affecting an estimated 10,000 babies born with severe deformities.
The non-chromatographic ChiraLig™ MRT™ process is applicable to a wide range of chiral separations including amino acids, amines, pharmaceutical drugs and intermediates, nutraceuticals, and agrochemicals. Significant time, space, labor, environmental and economic advantages are found using ChiraLig™ MRT™ products for Chiral separation services.
The ChiraLig™ MRT™ Process Provides Higher Purity at Lower Cost
- Low process operating costs due to fewer separation stages, simplicity of the process, and low consumption of operating chemicals
- Low capital costs due to reduced floor space, lower equipment cost, and decreased plant complexity
- Extension of drug intellectual property (IP) lifetime due to use of proprietary ChiraLig™ MRT™ chemistry and separation processes
- Reduction in the time required for drug introduction into the market due to high product throughput
- Operation using either aqueous or non-aqueous feed streams due to the versatility of the process
- Elimination of the need for downstream purification steps due to the combination of both enantio- and chemo-separations in one process step