Molecular Recognition Technology™ Makes Possible Circular Economy for Metals
MRT™ separation systems are available for extraction and recycling of a variety of metals from electronic waste (E-waste) and other spent secondary sources; acid mine drainage (AMD) streams, tailings and contaminated water supplies; and from product, process and associated waste streams. MRT™ systems efficiently recycle metals from these sources and recover them at high purity in a circular economy, supporting environmental remediation, ensuring metal sustainability and preventing metal leakage into the commons.
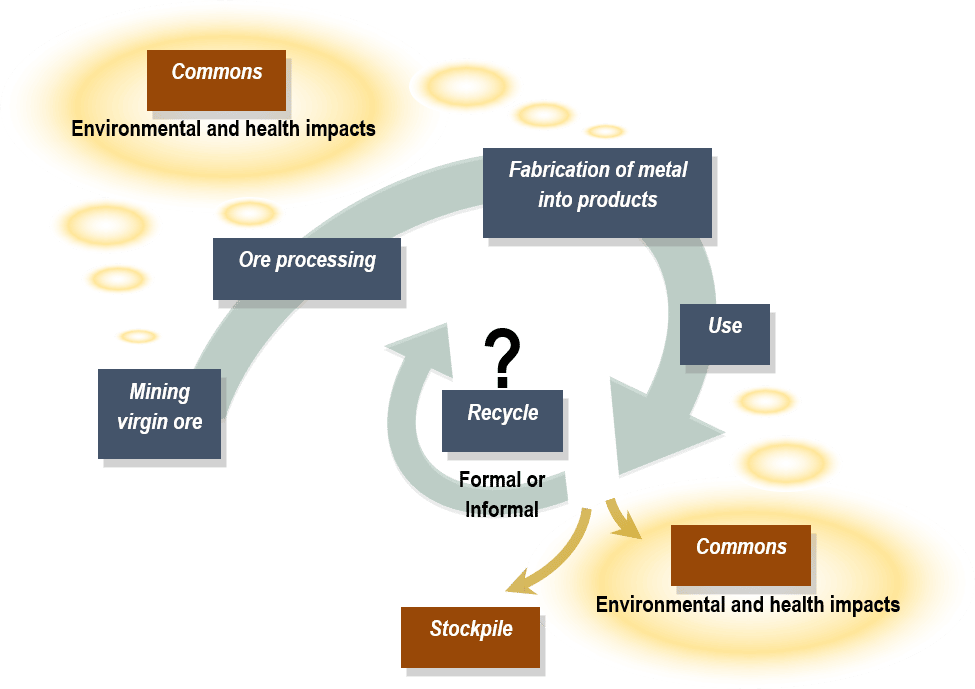
CAPTION: Metal flow from virgin ore to metal processing and product fabrication to end-of-life (EOL) with possible metal fates, including disposal (or leakage) to commons, temporary stockpiling, and formal or informal recycling.
SuperLig® MRT™ systems are unique in their ability to recover pure metals with high selectivity from complex matrices, even when the target metal is present at low concentrations. Concentration of target metals 100-fold or more in the recovery step makes MRT™ systems particularly valuable in processing streams where metal concentration levels are low. Conventional separation systems are inefficient and, hence, not cost-effective in recovering metals from such streams. A huge advantage of SuperLig® MRT™ systems is their ability to operate in a circular economy mode leading to metal sustainability and minimizing waste production since metals are recovered rather than discarded into the commons.
The following examples Illustrate MRT™ capabilities in a variety of applications.
- E-Waste
- Acid Mine Drainage (AMD)
- Toxic Metals
- Arsenic: Removal from Drinking Water
- Mercury: Removal for Purification of Metallurgical Sulfuric Acid
- Lead: Removal from Fly Ash
- Heavy Metals: Removal from Industrial Waste Streams
E-Waste
Cost-effective Recovery of Valuable and Toxic Metals from Electronic Waste
Electronic waste contains a large number of metals – precious, technology, base, toxic and others. IBC’s MRT™ processes efficiently recover individual target metals from E-waste at high purity in a circular economy mode, maintaining their sustainability and avoiding the generation of waste, which are highly desirable outcomes.
Global electronic waste is growing at an annual rate of approximately 50 million tons per year. This waste contains approximately 70 metals, mostly in small quantities in individual units. These metals represent a huge and valuable source of critical resources that are essentially all discarded, due in part to the lack of technologies capable of making required separations of individual metals either technologically or economically. Recycling rates of metals from E-waste are low, 1-15%, but must be greatly increased if metal sustainability is to be reached.
MRT™ processes are capable of making the separations needed to recover a wide range of metals from leachate solutions derived from spent E-waste products. For example, a green chemistry SuperLig® Molecular Recognition Technology™ (MRT™) process is used for the highly selective separation of palladium in the precious metals plant at the Boliden Rönnskär copper smelter. Boliden is a world leader in the recycling of E-waste.
The most valuable metal in E-waste is gold followed by palladium and other critical metals. Highly effective MRT™ processes are available to separate and recover at high purity gold, palladium, and other metals present at low concentrations in complex matrices.
Other examples of metal recovery from low concentration level solutions can be found throughout this website and are summarized as follows:
- Precious metals: gold, silver, platinum group metals
- Toxic metals: mercury, arsenic, lead, cadmium
- Specialty metals: indium, germanium, bismuth, rhenium, molybdenum
- Base metals: chromium, iron, manganese, nickel, copper, cobalt, zinc, tin
- Rare Earth Elements: All 17 individual rare earth elements (except Pm) or groups of rare earth elements
Benefits of the MRT™ process for separation and recovery of metals at low concentration levels such as are found in leachates of E-waste include:
- Highly selective separations of individual metals from complex matrices
- Exceptionally high recovery of the separated metal avoiding loss during processing
- Concentration of the target metal during elution by 100-fold or more enhancing effective recovery at low metal concentration levels
- Reduced capex and opex values due to efficient and effective MRT™ processes that separate and recover essentially all of the metal from the feed solution usually in a single pass, does not use organic solvents and generates minimal waste
Case Study: Highly Selective Separation and Recovery of Indium from Indium-Tin-Oxide (ITO) Scrap
Indium Tin Oxide (ITO: 90% In2O3, 10% SnO2) is an indispensable component of flat panel displays including liquid crystal displays. Up to 85% of global indium consumption is used in these displays. Worldwide indium primary resource supplies are inadequate to meet the increasing demand for indium in electronic displays as this market rapidly grows. Maintaining the sustainability of indium requires greatly increased recycling of it from E-waste since the recycling rate for indium is <1%.
Read our paper that describes an MRT™ process that efficiently separates tin and indium separately from ITO scrap. Pure indium was recovered by elution in concentrated form from the column at 15 g/L. Tin was recovered separately at high purity. Analysis of the eluate showed the following elements to be undetectable: tin, iron, copper, chromium, nickel, manganese, cobalt, aluminum, gallium, cadmium, magnesium, and zinc.
In a related study, an MRT™ process has been used for the selective recovery of indium from the etching waste produced during the patterning of indium on the ITO layer of flat-panel displays. (Hasegawa, H., et al. 2013, Microchemical J., 110, 133-139.)
Case Study: Gold Extraction and Recovery from Electroplating Solutions
Major uses of gold are in electronics, jewelry and arts. The MRT™ process enables single step recovery of potassium gold cyanide, KAu(CN)2, directly from spent plating solution and direct conversion to pure KAu(CN)2 or gold at 99.99% purity. This is a significant improvement over the number of steps required in conventional gold recovery processes. A further advantage of the MRT™ process is that it allows concurrent separation and recovery of silver. Both gold and silver are separated and recovered at 99.99% purity levels.
Acid Mine Drainage (AMD)
The use of MRT™ for treatment of AMD streams has demonstrated that high purity metals can be selectively extracted and recovered as products that can be sold for value in a sustainable, circular economy process.
Case Study: Recovery of Pure Copper from Acid Mine Drainage in a Recyclable, Circular Flowsheet
An MRT™ system was installed at an Asian open pit copper mining operation for copper extraction from an AMD stream. Copper was recovered as a high purity cathode product by electrowinning and the sulfuric acid regenerated in the electrowinning step was recycled.
Key Operational Parameters
- Positive rate of return based on the amount of copper recovered, and providing for opex and depreciation
- Feed flow rate: approximately 19,000 liters/minute
- Plant design capacity: one million kg/year of cathode copper
- Copper concentration in the feed: 100-400 ppm
- Feed solution pH: 3 to 5.5
- Copper concentration in the discharge effluent: less than five ppm
- Elution: mild (2 M) sulfuric acid, completed in less than two bed volumes
- Copper cathode production: high purity copper cathode produced by electrowinning the eluate, a copper electrolyte solution
- Sulfuric acid regenerated in electrowinning and recycled
Case Study: Acid Mine Drainage, Berkeley Pit, Montana, U.S.A.
Acid mine drainage sites are widespread in the U.S. and throughout the world. The largest of these in the U.S. is Berkeley Pit in Butte, MT. In a study funded by the U.S. Department of Energy, IBC installed a pilot plant to evaluate the effectiveness of MRT™ to separate copper, iron, aluminum, zinc, manganese, cadmium, and silver from solutions taken from Berkeley Pit. IBC successfully met all requirements of the study, which were to remove, separate, and recover the individual metal contaminants in sufficient purity for resale.
- aluminum sulfate - pulp and paper
- ferric sulfate - waste treatment
- zinc, copper, manganese, ferric sulfates - animal feed additives and fertilizer nutrients
- gypsum - cement production
Toxic Metals
SuperLig® products are effective in recovering and/or removing toxic metals to very low concentration levels in drinking water, as well as in product, process and waste streams. These elements are often present at low concentrations and cause serious human health and environmental damage. Their removal by traditional separation processes is difficult and expensive. Toxic elements need to be removed from product process streams as well as waste streams. For examples, read about the highly selective separation of cadmium from cobalt electrolyte, lead from electroless nickel plating waste solution and mercury from metallurgical sulfuric acid. Examples of the highly selective separation of toxic metals from a variety of streams using MRT™ are now presented.CASE STUDIES:
Selected examples of SuperLig® MRT™ separations for toxic and environmental metals:
Arsenic (As): Removal from Drinking Water
Access to clean water supplies is a major problem for a majority of the global population. Important water impurities include heavy metals and toxic metals, especially arsenic, lead, cadmium and mercury.
Hundreds of millions of people worldwide are exposed to unsafe levels of arsenic in their culinary water supplies. There is no safe arsenic exposure level in drinking water. Arsenic enters these supplies from its natural presence in groundwater, mineral processing plant emissions, and residues resulting from pollution associated with the use of arsenic-containing chemical products. Depending on conditions, arsenic may be present in trivalent, pentavalent, or methylated forms, complicating its separation and recovery. The trivalent form is ten times more toxic than the pentavalent form and 70 times more toxic than the methylated form (Rahman et al. 2011. Chemosphere, 82, 549-556). Water soluble inorganic arsenic species can be effectively separated and recovered at high purity using a combination of MRT™ products. Recovery in a circular economy is important because, despite its toxicity, arsenic is a valuable commodity that is usually discarded to the detriment of the environment when treatment is by conventional separation and recovery methods.
SAMPLE PROJECT: Selective Separation of Tri- and Penta-valent Arsenic Species from Aqueous Matrices using MRT™ Products
Rahman et al. have demonstrated a simple MRT™ process for separation of tri- and pentavalent arsenic species from an aqueous matrix. These authors state (Chemosphere, 82, 549-556, 2011) that “The process is rapid and is suitable for routine speciation analysis of inorganic arsenic in an aqueous matrix to comply with legislative recommendations in countries where the extent of contamination is severe, and a large number of samples are required to be monitored.” Samples studied included lake water and river water from local sources and spiked solutions. Recovery rates of arsenic(III) and arsenic(V) were approximately 100% with uncertainties of approximately ±1% in all cases. Measured total arsenic contents were in good agreement with certified values. The excellent selective separation and recovery at high purity are more remarkable considering that arsenic(III) exists as H3AsO3 and As(V) as H2AsO4-/ HAsO42- in the pH range studied.
Benefits of the MRT™ process include:
- Selective single-step separation of arsenic(III) and arsenic(V) with a single MRT™ column
- Easy operation, rapid separation performance, and reusability of the resin for more than 100 cycles without loss of performance
- Demonstration that the MRT™ process is a suitable and economic option for arsenic removal from natural waters
Mercury (Hg): Removal for Purification of Metallurgical Sulfuric Acid
Mercury is widely found in zinc, lead, copper, gold and manganese sulfide ores, is broadly dispersed during mining activities and is a major toxic product from the combustion of coal. SuperLig® 88 is very effective in separating Hg from contaminated solutions. A commercial example is the use of SuperLig® 88 to remove mercury impurity from concentrated sulfuric acid that is a by-product of processing sulfide ores.
 SAMPLE PROJECT: A large-scale commercial plant for removal of mercury from sulfuric acid was installed at Britannia Zinc, Ltd.
The initial plant capacity was 25,000 metric tons of concentrated sulfuric acid per year. The mercury content of the acid was 100 mg/L. The system flow rate was variable from 50 to 1,000 m3 per hour, depending on requirements. The output sulfuric acid contained <0.1 mg/L (ppm) Hg.
Advantages of the MRT™ SuperLig® 88 system can be summarized as follows:
SAMPLE PROJECT: A large-scale commercial plant for removal of mercury from sulfuric acid was installed at Britannia Zinc, Ltd.
The initial plant capacity was 25,000 metric tons of concentrated sulfuric acid per year. The mercury content of the acid was 100 mg/L. The system flow rate was variable from 50 to 1,000 m3 per hour, depending on requirements. The output sulfuric acid contained <0.1 mg/L (ppm) Hg.
Advantages of the MRT™ SuperLig® 88 system can be summarized as follows:
- Large and high capital cost gas handling equipment is avoided, as the MRT™ system treats the liquid phase acid product
- The MRT™ system can achieve sustainable levels of mercury at the <0.1 mg/L concentration level in sulfuric acid solutions, which cannot be assured by other processes.
- The recovered Hg product can be marketed or disposed of in an environmentally safe manner avoiding unsafe discharge of mercury to the commons
- The compact size of the MRT™ system assures that it can be added to an existing plant or designed into a new plant where the plant area is restricted
- Power and maintenance costs for the MRT™ system are very low
- MRT™ systems can be easily and economically expanded: an MRT™ system can be installed to treat a portion of the sulfuric acid and expanded as needed to treat additional sulfuric acid, thereby greatly reducing capital and operating expenses
Lead (Pb): Removal from Fly Ash
Fly ash resulting from incineration of refuse wastes often contains a variety of metals, including toxic lead, cadmium and arsenic. SuperLig® 141 is highly selective for lead.
SAMPLE PROJECT
In a pilot plant operation for Takuma Co. (Japan), the selective removal of lead from a feed solution containing lead, iron, aluminum, copper, zinc, cadmium, arsenic and antimony was accomplished using MRT™. In this system, only lead was retained on the SuperLig® column with the remaining metals passing to raffinate. The lead was recovered in pure form at 100% yield. SuperLig® MRT™ systems provide a clean chemistry means for selectively separating and recovering lead at high purity from contaminated solutions.
Heavy Metals: Removal from Industrial Waste Streams
- Copper (Cu)
- Cadmium (Cd)
- Chromium (Cr III, VI)
- Nickel (Ni)
- Lead (Pb)
- Zinc (Zn)
- Silver (Ag)
- Removal of these metals was much more effective using MRT™ than conventional methods
- Cost analysis was favorable for MRT™ over conventional systems
- The payback period was favorable for MRT™ installed as a replacement system
- The footprint of the MRT™ system was small and ancillary equipment minimal
Expert Reviews
Rahman, I.M.M., et al. 2013. Selective Separation of Tri- and Pentavalent Arsenic in Aqueous Matrix with a Macrocycle-immobilized Solid-Phase Extraction System, Water, Air, and Soil Pollution, 234, 1526-1537.
“The [MRT™] process offers a single-step separation option of trivalent and pentavalent arsenic species that commonly exist in the natural aqueous matrix. Easy operation, rapid separation performance, and reusability … make it a suitable and economic option for, particularly, the arsenic-prone nations suffering from natural arsenic contamination.”
Rahman, I.M.M., et al. 2011. Selective Separation of Arsenic Species from Aqueous Solutions with Immobilized Macrocyclic Material Containing Solid Phase Extraction Columns, Chemosphere, 82, 549-556.
“It is possible to overcome the tedious preconcentration process by following the proposed selective separation technique.”
“Easy operation, virtually unlimited loading and elution capability of the sorbent material without losing the analytical performance and high-sensitive separation ability can make the proposed technique a useful one for selective separation of arsenic species from natural waters.”